Water electrolysis and fuel cells: how hydrogen is produced and used
Introduction
Hydrogen is considered a key pillar in the energy transition toward a sustainable future.
It can be produced from renewable sources and used to generate energy with zero emissions.
Two core technologies in this process are water electrolysis and fuel cells.
Below is a clear and detailed explanation of how they work, the available technologies, and their role in the modern energy system.
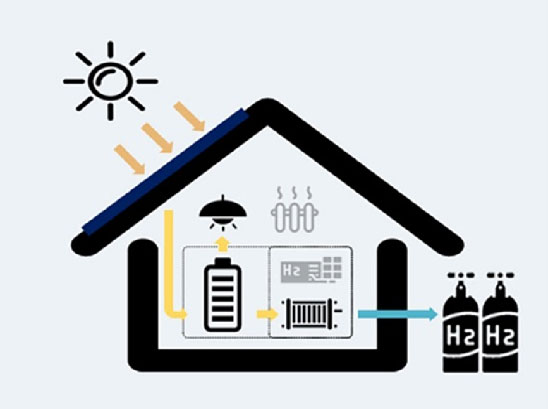
1. Water electrolysis: generating hydrogen with solar energy
Electrolysis is a chemical process that uses electricity to split water (H₂O) into its basic elements: hydrogen (H₂) and oxygen (O₂).
When the electricity comes from renewable sources like solar power, the hydrogen produced is called green hydrogen.
The process takes place inside a device called an 'electrolyzer'.
There are different types of electrolyzers, each one with specific features.
1.1 Alkaline Electrolyzers (AWE – Alkaline Water Electrolysis)
These are the most technologically well-established. They use an alkaline solution (typically potassium hydroxide, KOH) as the electrolyte.
They are reliable and affordable but have slower response times and are best suited for constant loads.
1.2 Proton Exchange Membrane Electrolyzers (PEM)
PEM electrolyzers are more compact and respond quickly to load variations.
They use a solid polymer membrane as the electrolyte.
They are ideal for variable renewable sources (like solar power),but they are more expensive due to the use of noble materials (e.g. platinum).
1.3 Solid Oxide Electrolyzers (SOEC – Solid Oxide Electrolysis Cell)
These operate at high temperatures (700–1000°C), using ceramic oxides as the electrolyte.
They offer very high theoretical efficiency, especially when integrated into industrial processes where heat is available.
They are still under development.
1.4 Anion Exchange Membrane Electrolyzers (AEM)
An emerging technology that tries to combine the benefits of alkaline systems (low cost) and PEM systems (fast response), using membranes that transport anions instead of protons. It's still in the testing and optimization phase.
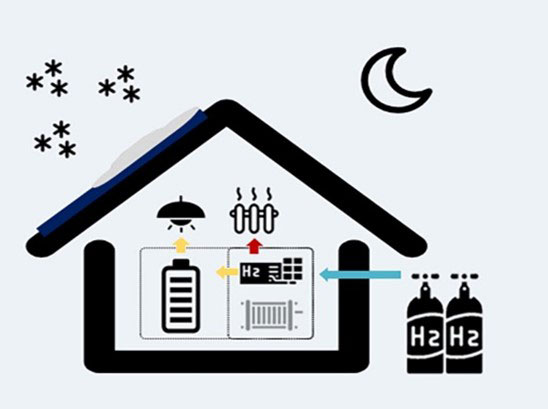
2. Fuel Cells: Converting Hydrogen into Electricity
A fuel cell is an electrochemical device that converts the chemical energy of hydrogen into electrical energy. This process takes place by combining hydrogen and oxygen in a controlled manner, generating electricity, heat and water as the only byproduct.
2.1 How does a fuel cell work?
Inside the cell:
- Hydrogen (H₂) enters the anode, where it is splitted into protons (H⁺) and electrons (e⁻)
- Protons pass through the electrolyte membrane to the cathode
- Electrons travel through an external circuit, generating electric current
- At the cathode, protons, electrons, and oxygen (O₂) recombine to form water (H₂O)
2.2 Types of Fuel Cells
Fuel cells also differ based on the technology used. The main types are:
- PEMFC (Proton Exchange Membrane Fuel Cell): the most widespread, used in vehicles and residential applications
- SOFC (Solid Oxide Fuel Cell): operate at high temperatures, suitable for stationary applications
- PAFC (Phosphoric Acid Fuel Cell): use phosphoric acid as electrolyte, for stationary use
- MCFC (Molten Carbonate Fuel Cell): high-temperature cells used in industrial plants
- AFC (Alkaline Fuel Cell): one of the earliest technologies, also used in space missions, but sensitive to CO₂
3. Electrolysis vs Fuel Cells
The full hydrogen production and use cycle includes:
- Hydrogen production (via electrolysis)
- Storage and transport (e.g. in cylinders)
- Electricity generation (via fuel cells)
There are efficiency losses in both phases, but the flexibility and sustainability of the system make hydrogen a valuable energy resource for the future.
Conclusion
Hydrogen, produced through electrolysis and used in fuel cells, represents a concrete solution for clean and safe energy.
Today, the technologies available are various and continuously evolving, with room for improvement in cost, efficiency, and scalability.
Integrating these systems with renewable sources like solar energy enables a zero-emission virtuous cycle.
To tackle global climate challenges, large-scale adoption of these technologies has become essential today.